- Part 1. What is a LiCoO₂ battery?
- Part 2. LiCoO₂ battery reaction: how it works
- Part 3. Advantages and limitations of lithium cobalt oxide batteries
- Part 4. The cobalt challenge and industry response
- Part 5. Typical applications of LiCoO₂ batteries
- Part 6. LiCoO₂ vs other lithium-ion chemistries
- Part 7. How to extend LiCoO₂ battery life
- Part 8. LiCoO₂ battery FAQs
- Part 9. Key takeaways
LiCoO₂ (commonly searched as “licoo2 battery” or lithium cobalt oxide battery) is one of the earliest and most widely used lithium-ion cathode chemistries. With a practical energy density of 150–200 Wh/kg and stable 3.7 V nominal output, it remains a core solution for compact, high-performance power systems. This guide explains how LiCoO₂ batteries work, the underlying electrochemical reaction, real-world applications, and how engineers should evaluate LiCoO₂ versus newer lithium-ion chemistries in 2026.
Part 1. What is a LiCoO₂ battery?
A LiCoO₂ battery is a rechargeable lithium-ion battery that uses lithium cobalt oxide (LiCoO₂) as its cathode material and graphite as the anode. It was commercialized by Sony in the early 1990s and set the foundation for modern lithium-ion cells.
Key defining characteristics include:
- High gravimetric energy density for space-constrained designs
- Stable discharge voltage around 3.7 V
- Mature manufacturing ecosystem and predictable performance
Today, LiCoO₂ chemistry is still dominant in consumer electronics and selected medical and industrial devices, even as EV platforms increasingly migrate to NMC or LFP chemistries.
Part 2. LiCoO₂ battery reaction: how it works
Understanding the LiCoO₂ battery reaction is essential for correct system design and lifecycle optimization.
Electrochemical principle
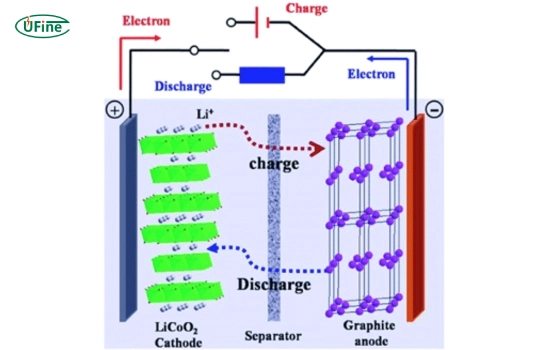
During charging, lithium ions are extracted (de-intercalated) from the LiCoO₂ cathode and migrate through the electrolyte to the graphite anode. During discharge, the reaction reverses and electrical energy is released.
Overall reaction (simplified):
- Charge: LiCoO₂ → Li₁₋ₓCoO₂ + xLi⁺ + xe⁻
- Discharge: Li₁₋ₓCoO₂ + xLi⁺ + xe⁻ → LiCoO₂
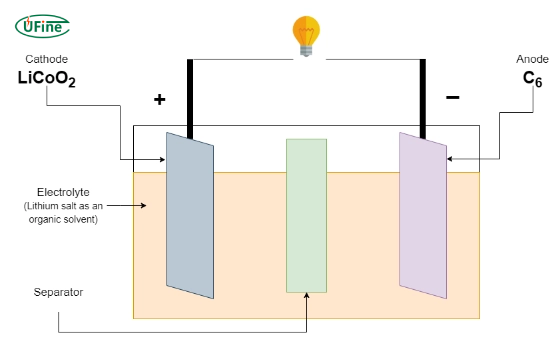
Cell components
- Cathode: Lithium cobalt oxide (LiCoO₂)
- Anode: Graphite (C₆)
- Electrolyte: Lithium salt (e.g., LiPF₆) in organic solvents
- Separator: Microporous polymer preventing short circuits
Engineering note: Excessive delithiation (> 50%) destabilizes the LiCoO₂ crystal lattice, which is why strict upper-voltage control (4.2 V max) and a reliable BMS are mandatory.
Part 3. Advantages and limitations of lithium cobalt oxide batteries
Advantages
- High Energy Density: Ideal for compact devices where volume and weight are critical
- Stable Voltage Output: Consistent power delivery for sensitive electronics
- Mature Supply Chain: Predictable quality and well-understood aging behavior
- Good Manufacturability: High yield and uniform cell performance
Limitations
- Thermal Stability: Lower safety margin compared with LiFePO₄; requires robust protection circuits
- Cycle Life: Typically 500–1,500 cycles depending on depth of discharge
- Cobalt Dependency: Higher material cost and ethical sourcing concerns
- Moderate Power Capability: Not optimized for high-C-rate or fast-charge designs
Part 4. The cobalt challenge and industry response
Cobalt sourcing remains a strategic and regulatory concern. According to IEA and NGO reports, a significant share of global cobalt supply originates from the DRC, increasing ESG pressure on OEMs.
Industry responses include:
- Reduced-Cobalt Chemistries: NMC and NCA formulations with cobalt content below 10%
- Closed-Loop Recycling: Hydrometallurgical processes recovering > 90% cobalt (e.g., Umicore)
- Material Innovation: Solid-state and cobalt-free cathode research programs
For background standards and sustainability references, see the International Energy Agency critical minerals report.
Part 5. Typical applications of LiCoO₂ batteries
LiCoO₂ batteries are best suited for applications prioritizing energy density over extreme cycle life or abuse tolerance:
- Consumer Electronics: Smartphones, laptops, tablets, wearables
- Medical Devices: Infusion pumps, diagnostic equipment, portable monitors
- Industrial Instruments: Test equipment, handheld analyzers
- Early-Generation EV Packs: Historically used before large-scale NMC adoption
For energy-storage-focused systems, see internal comparison with LiFePO₄ battery technology.
Part 6. LiCoO₂ vs other lithium-ion chemistries
Technical comparison overview
| Feature | LiCoO₂ | LiFePO₄ | NMC 811 |
|---|---|---|---|
| Energy Density | 150–200 Wh/kg | 90–120 Wh/kg | 220–280 Wh/kg |
| Cycle Life | 500–1,500 | 2,000+ | 800–1,200 |
| Thermal Stability | Moderate | High | Moderate |
| Cost | High | Moderate | High |
| Typical Use Case | Compact electronics | Energy storage, ESS | EV traction |
Selection guidance
- Choose LiCoO₂ when space and weight dominate design constraints
- Choose LiFePO₄ for long-life, safety-critical systems
- Choose NMC for high-energy EV or industrial traction platforms
Part 7. How to extend LiCoO₂ battery life
From an engineering perspective, lifespan is primarily limited by voltage stress and temperature.
Best practices include:
- Avoid sustained charging above 4.2 V
- Operate between 10 °C and 35 °C whenever possible
- Store at 40–60% SOC for long idle periods
- Limit deep discharge below 3.0 V
- Use a calibrated BMS with accurate cell balancing
Part 8. LiCoO₂ battery FAQs
What is the typical lifespan of a LiCoO₂ battery?
Most LiCoO₂ batteries deliver 500–1,500 cycles, depending on depth of discharge, temperature, and charge voltage.
Are LiCoO₂ batteries still used in electric vehicles?
They are no longer mainstream in modern EV packs but were used in early designs and remain relevant in high-energy auxiliary systems.
What voltage does a LiCoO₂ battery operate at?
Nominal voltage is 3.7 V, with a typical operating range of 3.0–4.2 V.
How can I tell if a LiCoO₂ battery is degrading?
Capacity loss below 80%, abnormal heating, swelling, or increased internal resistance are common indicators.
Part 9. Key takeaways
- LiCoO₂ batteries offer one of the highest energy densities among commercial lithium-ion chemistries, making them ideal for compact devices.
- The LiCoO₂ battery reaction relies on reversible lithium intercalation, requiring strict voltage and thermal control.
- Compared with LiFePO₄, lithium cobalt oxide batteries trade safety and cycle life for higher energy density.
- Cobalt cost and sourcing risks are the primary long-term limitations of LiCoO₂ technology.
- Proper charge voltage management and temperature control can significantly extend LiCoO₂ battery service life.
Related Tags:
More Articles

8 Volt Golf Cart Batteries: Tips, Types & Lifespan
Explore 8 volt golf cart batteries, types, lifespan, and maintenance tips. Find out which battery suits your cart best in 2026.
What Is the Charge Voltage of the AGM Battery?
Discover the ideal charge voltage for AGM batteries. Ensure optimal performance and longevity. Learn more about maintaining your battery today!
Bad Battery Cell Symptoms: How to Tell If a Battery Cell Is Dead
Learn how bad battery cells behave, how to test them, and when replacement is the safest option.
18650 Maximum Capacity in 2026: What’s Real and What’s Hype?
Discover the real 18650 battery capacity limits in 2026, top high-capacity cells, and how to avoid fake claims.
Lithium Battery Sizes Guide: 18650, 21700, LiPo & Coin
Learn about lithium battery sizes, form factors, and uses. Compare dimensions and capacities for cylindrical, pouch, prismatic, and more.