Batteries are essential in many devices and systems, from smartphones to electric vehicles. However, battery performance can be significantly affected by extreme temperatures. This article will explore the effects of high and low temperatures on battery performance and the best practices for charging and discharging batteries under such conditions.
Part 1. Effect of high temperature on battery performance
Increased Internal Resistance
- High temperatures can cause the materials inside batteries to react differently, increasing internal resistance.
- This increased resistance makes it harder for electricity to flow smoothly within the battery, reducing efficiency and capacity.
- The heat-induced degradation of battery components, including electrolytes and electrodes, increases internal resistance.
- Electrolytes may evaporate or decompose at high temperatures, altering their chemical composition and hindering ion movement.
- Similarly, electrodes may undergo structural changes or chemical reactions that impede their performance, further increasing internal resistance.
Accelerated Aging and Degradation
- Batteries exposed to high temperatures undergo accelerated aging, causing them to degrade more rapidly.
- This accelerated aging process leads to diminished capacity, shortened cycle life, and overall deterioration in performance.
- Common signs of battery degradation due to high-temperature exposure include swelling, leakage, and reduced charge retention.
- Swelling occurs as battery components expand, compromising the integrity of the casing and potentially leading to electrolyte leakage.
- Leakage exacerbates degradation by causing chemical reactions and corrosion within the battery.
- Additionally, batteries exposed to high temperatures may experience reduced charge retention, meaning they lose their charge more quickly even when not in use, further limiting their usability and lifespan.
Part 2. Effect of low temperature on battery performance
Reduced Electrochemical Activity
- Low temperatures hinder electrochemical reactions in batteries, lowering their capacity and power output.
- “Cold cracking” occurs in battery electrodes due to low temperatures, leading to reduced performance.
- Cold temperatures slow the movement of ions and electrons within the battery, diminishing its ability to generate and store energy efficiently.
Increased Internal Resistance
- Low temperatures increase the internal resistance of batteries, making it harder for electricity to flow smoothly.
- This increased resistance limits the battery’s ability to deliver power efficiently, resulting in reduced performance.
- Cold starting in automotive batteries becomes challenging due to increased internal resistance, leading to difficulty starting the vehicle in cold weather.
- Other applications also face challenges with cold starting or operation in low temperatures due to increased internal resistance and reduced power output.
Part 3. Charging at high and low temperatures
High temperature
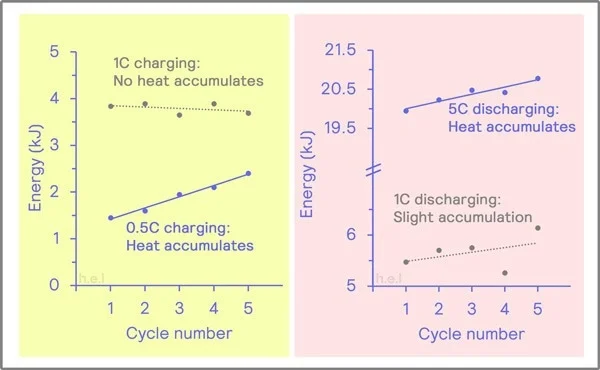
- Charging batteries at high temperatures can lead to accelerated chemical reactions within the battery, resulting in faster charging times.
- However, high temperatures can also increase the risk of overheating, which may damage the battery and reduce its lifespan.
- Heat can cause the electrolyte in the battery to evaporate more quickly, leading to a loss of electrolyte and potential damage to the battery’s internal components.
- Additionally, high temperatures can increase the battery’s internal resistance, reducing its efficiency and overall charging performance.
- It’s essential to monitor the battery’s temperature during charging and avoid exposing it to excessively high temperatures to prevent damage and ensure optimal charging efficiency.
Low temperature
- Charging batteries at low temperatures can slow down chemical reactions within the battery, resulting in longer charging times.
- Cold temperatures can also increase the viscosity of the electrolyte, making it more difficult for ions to move freely within the battery and reducing charging efficiency.
- Charging batteries in frigid temperatures can cause the electrolyte to freeze, damaging the battery and preventing it from charging correctly.
- Also, low temperatures can increase the risk of overcharging, as the battery’s capacity may appear lower than it is due to slower chemical reactions.
- To mitigate the effects of low temperatures on charging, using a battery warmer or preheating the battery before charging is recommended to ensure optimal performance and prevent damage.
Part 4. Discharging at high and low temperatures
High temperature
- Discharging batteries at high temperatures can lead to increased chemical activity within the battery, allowing for faster discharge rates.
- However, high temperatures can also accelerate the degradation of battery components, leading to reduced capacity and overall performance.
- Heat can cause the electrolyte to evaporate more quickly, potentially leading to a loss of electrolyte and damage to the battery’s internal structure.
- Additionally, high temperatures can increase the battery’s internal resistance, reducing its efficiency and causing voltage drops during discharge.
- It’s essential to monitor the battery’s temperature during discharge to prevent overheating and minimize the risk of damage to the battery.
Low temperature
- Discharging batteries at low temperatures can slow down chemical reactions within the battery, resulting in decreased discharge rates.
- Cold temperatures can increase the viscosity of the electrolyte, making it more difficult for ions to move freely within the battery and reducing discharge efficiency.
- Discharging batteries in frigid temperatures can cause the electrolyte to freeze, preventing the battery from discharging potentially and adequately damaging it.
- Additionally, low temperatures can reduce the battery’s overall capacity, as the chemical reactions may proceed less efficiently.
- To mitigate the effects of low temperatures on discharge, using insulated battery packs and avoiding exposing the battery to icy conditions whenever possible are essential measures.
Part 5. Lithium-ion charging and discharging temperature optimization
Charging temperature optimization
- The ideal charging temperature range for lithium-ion batteries is typically between 0°C and 45°C (32°F to 113°F).
- Charging at temperatures outside this range can lead to reduced charging efficiency and potential damage to the battery.
- Charging below 0°C (32°F) can cause lithium plating on the battery’s anode, deleting battery performance and safety.
- Conversely, charging at temperatures above 45°C (113°F) can accelerate the degradation of the battery, leading to reduced capacity and lifespan.
- It’s essential to monitor the battery’s temperature during charging and avoid exposing it to extreme temperatures to ensure optimal performance and safety.
Discharging temperature optimization
- The ideal discharging temperature range for lithium-ion batteries is also typically between 0°C and 45°C (32°F to 113°F).
- Discharging at temperatures outside this range can result in decreased discharge efficiency and potential damage to the battery.
- Discharging at temperatures below 0°C (32°F) can cause the battery’s capacity to drop significantly. It may even result in the battery becoming temporarily unusable.
- Discharging at temperatures above 45°C (113°F) can accelerate the degradation of the battery, leading to reduced capacity and lifespan.
- To optimize the discharging temperature, using insulated battery packs and avoiding exposing the battery to extreme temperatures whenever possible are essential measures.
Part 6. Conclusion
Extreme temperatures have a significant impact on battery performance. High temperatures accelerate battery degradation, increase self-discharge, and reduce capacity. In contrast, low temperatures slow chemical reactions and decrease the battery’s capacity and voltage output. To ensure optimal battery performance, avoiding exposure to extreme temperatures is crucial whenever possible.
Related Tags:
More Articles

Comprehensive Guide to Choosing the Right Cart Battery
Choosing the right cart battery ensures optimal performance and longevity. This guide covers cart battery types and helps you make an informed choice.
The Ultimate Guide to 18650 Button Top Battery
18650 button top batteries are popular for their high energy density and reliability. This guide covers their key features, usage, and maintenance tips.
The Power of Slim: Unveiling the Potential of Flat Lithium Ion Battery
Flat lithium-ion batteries power devices from phones to vehicles. This article explores their design, benefits, types, applications, charging, and safety.
The Comprehensive Guide to Battery Balancing and Battery Balancer
Battery balancing and balancers optimize performance, longevity, and safety. This guide covers techniques and tips for choosing the right balancer.
10 Key Facts About Drone Battery for 2024
Uncover crucial insights with "10 Key Facts About Drone Battery for 2024." Learn the latest trends and essential details on drone batteries.