
- Part 1. Atomic structure & basic characteristics
- Part 2. Physical properties of lithium
- Part 3. Thermal and electrical conductivity
- Part 4. Chemical reactivity & compounds
- Part 5. Isotopes and nuclear properties
- Part 6. Electrochemical properties & battery relevance
- Part 7. Applications & industrial uses
- Part 8. Environmental & health considerations
- Part 9. Strategic importance & sustainability
- Part 10. Future trends and research directions
Lithium is a silvery-white alkali metal and the lightest solid element in the periodic table. It has captured global attention not only for its unique scientific properties but also for its critical role in powering the modern world. From electronics to green energy, lithium’s properties define its significance across industries. Understanding its physical, chemical, and electrochemical traits is essential for both researchers and businesses seeking sustainable solutions.
Part 1. Atomic structure & basic characteristics
Lithium has an atomic number of 3, placing it in Group 1 of the periodic table with other alkali metals such as sodium and potassium. Its electronic configuration is 1s² 2s¹, meaning it has a single valence electron in the outer shell. This lone electron is responsible for its high reactivity and its tendency to form ionic compounds.
- Atomic number: 3
- Relative atomic mass: ~6.94
- Valency: +1
One unique feature of lithium is its diagonal relationship with magnesium. Despite being in different groups, lithium and magnesium share similarities such as forming covalent compounds and having comparable ionic radii. This relationship influences lithium’s distinct behavior compared to other alkali metals.
Part 2. Physical properties of lithium
Lithium is the least dense metal, with a density of only 0.534 g/cm³—so light it can float on water. Its physical properties stand out among metals:
- Appearance: Silvery-white, soft, and easily cut with a knife.
- Melting point: 180.5 °C (one of the lowest among metals).
- Boiling point: 1,342 °C.
- Specific heat capacity: Highest among solid elements, allowing it to absorb heat efficiently.
These properties make lithium valuable in thermal regulation applications and alloys that benefit from its lightweight and heat management characteristics.
Part 3. Thermal and electrical conductivity
Lithium is an excellent conductor of both heat and electricity. Its high thermal conductivity allows it to dissipate heat quickly, while its electrical conductivity enables efficient current flow. These traits are crucial in advanced technologies such as energy storage systems and cooling applications. In comparison to heavier metals, lithium offers the unique advantage of combining conductivity with extremely low weight, which is why it is indispensable in compact, high-performance designs.
Part 4. Chemical reactivity & compounds
Lithium is highly reactive, though less so than sodium or potassium. It tarnishes quickly in air due to the formation of lithium oxide and reacts readily with water to form lithium hydroxide and hydrogen gas.
Key reactions include:
- With water: Produces lithium hydroxide (LiOH), a strong base.
- With halogens: Forms lithium halides such as lithium chloride (LiCl).
- With nitrogen: Produces lithium nitride (Li₃N), a unique feature among alkali metals.
Common lithium compounds include:
- Lithium carbonate (Li₂CO₃): Widely used in medicine and ceramics.
- Lithium hydroxide (LiOH): Applied in air purification systems and batteries.
- Lithium chloride (LiCl): A hygroscopic salt used in drying processes.
The wide chemical reactivity of lithium underpins its industrial versatility.
Part 5. Isotopes and nuclear properties
Lithium naturally occurs as two stable isotopes: lithium-6 (Li-6) and lithium-7 (Li-7). Li-7 is the most abundant, accounting for about 92.5% of natural lithium, while Li-6 makes up the remainder.
Both isotopes are important in nuclear technology:
- Li-6 is used to produce tritium, a fuel in nuclear fusion reactions.
- Li-7 plays a role in cooling systems for nuclear reactors, thanks to its ability to absorb heat while remaining stable.
These nuclear properties add strategic value to lithium beyond its common industrial uses.
Part 6. Electrochemical properties & battery relevance
Lithium’s electrochemical potential is the highest among all metals, making it ideal for energy storage. It can release a large amount of energy per unit mass, which is why lithium-ion batteries dominate consumer electronics and electric vehicles.
Key properties:
- High electrode potential: Enables lightweight, high-energy batteries.
- Small ionic radius: Facilitates rapid movement of Li⁺ ions within electrolytes.
- Formation of SEI (Solid Electrolyte Interphase): Protects battery electrodes and ensures long cycle life.
Lithium’s ability to reversibly intercalate into host materials, such as graphite and layered oxides, underpins modern rechargeable battery technology. This electrochemical versatility makes lithium the cornerstone of the global energy transition.
Part 7. Applications & industrial uses
Lithium’s unique properties translate into a wide range of practical applications:
- Batteries: Lithium-ion and lithium-polymer batteries power smartphones, laptops, electric vehicles, and energy storage systems.
- Alloys: Lithium-aluminum alloys reduce weight in aerospace and automotive structures while maintaining strength.
- Glass and Ceramics: Lithium compounds improve thermal resistance in specialty glass and ceramics.
- Lubricants: Lithium stearate and lithium hydroxide are used in high-temperature greases.
- Air Treatment: Lithium chloride and lithium bromide serve as drying agents in air conditioning and industrial dehumidification.
- Pharmaceuticals: Lithium carbonate is an established mood stabilizer for treating bipolar disorder.
Its versatility makes lithium a metal of immense industrial and medical importance.
Part 8. Environmental & health considerations
While lithium is indispensable, its handling requires care.
Environmental impacts:
- Mining lithium often requires large volumes of water, especially in arid regions, leading to ecological stress.
- Improper disposal of lithium batteries can release toxic substances into soil and water.
Health impacts:
- Lithium compounds are corrosive and can cause burns upon contact.
- Inhalation of lithium dust or prolonged exposure may cause respiratory irritation and other health effects.
- In medical use, lithium therapy must be carefully monitored to prevent toxicity.
These considerations highlight the importance of safe handling, sustainable mining, and effective recycling systems.
Part 9. Strategic importance & sustainability
Lithium has emerged as a strategic resource in the global shift toward renewable energy. Its role in electric vehicles and grid-scale energy storage makes it central to decarbonization strategies. However, the rapid growth of demand raises concerns about sustainability.
Key sustainability issues:
- Resource scarcity: Concentrated supply in regions such as South America and Australia creates geopolitical challenges.
- Water usage: Extraction from salt flats consumes significant water, impacting local ecosystems.
- Recycling challenges: Lithium recycling technologies are still developing, and scaling them is critical for long-term supply security.
Sustainable approaches include improving recycling efficiency, investing in alternative battery chemistries, and developing greener extraction methods. The balance between demand growth and responsible sourcing will shape lithium’s role in the energy future.
Part 10. Future trends and research directions
Ongoing research continues to expand the potential of lithium. Areas of focus include:
- Solid-state batteries: Offering higher energy density and improved safety compared to conventional lithium-ion cells.
- Next-generation electrolytes: Exploring glyme-based and other advanced systems for better stability.
- Superconductivity: Under extreme pressures, lithium demonstrates fascinating superconducting properties.
- Fusion technology: Lithium’s role in producing tritium highlights its relevance to future clean energy solutions.
The future of lithium is not limited to existing applications; its properties make it a promising candidate for groundbreaking technologies.
Related Tags:
More Articles
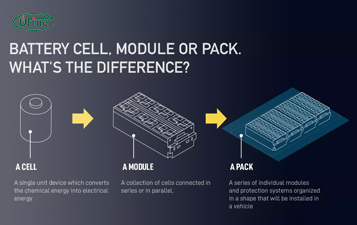
What is the Difference Between Battery Cell, Battery Control Module, and Battery Pack?
Compare battery cells, modules, and packs. Learn functions, design differences, control modules, and selection tips for EV, ESS, and industrial use.
How to Prevent LiPo Battery Explosion?
Can LiPo batteries explode or catch fire? Learn key causes of LiPo battery fires and proven charging, storage, and handling tips to reduce explosion risk.
Aluminium Ion Battery vs Lithium-Ion: A Detailed Comparison
Compare aluminium ion battery vs lithium-ion battery in energy density, charging speed, safety, cost, and uses. A practical guide for engineers and buyers.
C vs D vs AA Battery: Size, Voltage, Capacity & Key Differences Explained
Compare AA, C, and D batteries by size, voltage, capacity, and lifespan. Learn the real difference between C and D batteries and which one you should use.
What is a battery MSDS? Learn what a lithium battery MSDS certificate includes, why it’s required for shipping and compliance, and how to read it correctly.



