
If you’ve ever worked with batteries, chances are you’ve asked yourself this question:
Is the anode positive or negative? What about the cathode?
In fact, anode vs cathode is one of the most commonly misunderstood topics in battery technology—especially in lithium-ion batteries. The confusion mainly comes from the fact that their roles change depending on whether the battery is charging or discharging.
In this guide, you’ll learn exactly what a battery anode and cathode are, how they differ, and how to correctly identify the positive and negative electrodes.
Key Takeaways
- During discharge, the anode is negative and the cathode is positive
- During charging, their roles reverse
- Anode and cathode describe electrochemical behavior, not fixed polarity
- In lithium-ion batteries, material choice directly affects performance, safety, and lifespan
Part 1. Anode and cathode definition
If you are a beginner and want to know what an anode and cathode are, you are at the right place. So, here is their description.
This is the part most people care about—and where most mistakes happen.
If you want to understand how a battery works from start to finish, you can check out our detailed guide on how a battery works.
The short answer (bookmark this):
When a battery is discharging:
- Anode → Negative
- Cathode → Positive
When a battery is charging:
- Anode → Positive
- Cathode → Negative
Why does this happen? Because anode and cathode are defined by reaction type, not by fixed electrical polarity.
During discharge, electrons flow from the anode to the cathode through the external circuit, powering your device. When charging, an external power source forces electrons to flow in the opposite direction, effectively flipping their roles.
To see a clear scientific explanation of oxidation and reduction, you can refer to Encyclopaedia Britannica.
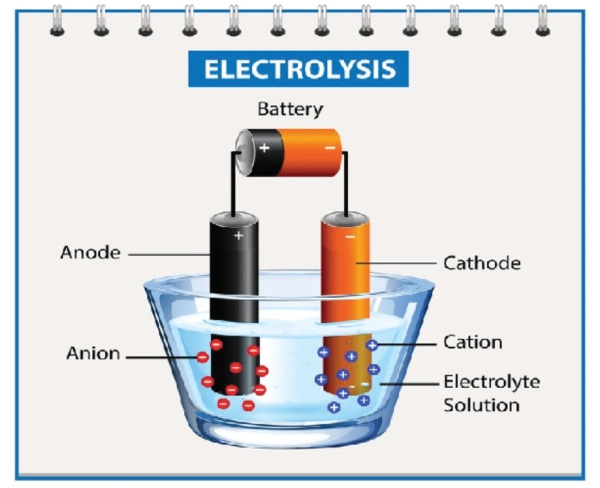
Cathodes and Anodes are electrodes of any battery or electrochemical cell. These help in the flow of electrical charges inside the battery.
Moreover, the cathode has a positive charge, where reduction occurs (receives electrons).
In contrast, the anode has a negative charge, where oxidation occurs (loss of electrons) and electricity is produced. On the other hand, if we talk about the charging process of a battery, electrons flow from the cathode to the anode (reverse order), storing energy that can later be used to power devices.
So, the following is a description to help you understand the anode and cathode and how they work in any battery or electrochemical cell.
What is a Battery Anode?
The anode is one of the essential components of the battery. It is a negative electrode which is immersed in an electrolyte solution. So, when the current is allowed to pass through the battery, it oxidizes itself, and the negative charges start to lose and travel towards the positive electrode.
What is the Battery Cathode?
In contrast to the anode, the cathode is a positive electrode of the battery. It gets electrons and is reduced itself. Moreover, the cathode is immersed in the battery’s electrolyte solution. So, when the current is allowed to pass, the negative charges move from the anode side and reach the cathode.
The cathode gains these negatively charged electrons. Thus, it reduces itself. This constant flow of negative charges towards positive ones helps generate electricity via batteries. Electrolyte solution is bridging in transmitting electrons from one electrode to another.
Part 2. Battery anode vs cathode: What’s the difference?
The cathode and anode of the battery are of different types. Thus, they serve for a different and unique purpose. So, here is a detailed aspect of how the anode and cathode and anode of a battery have different natures from each other;
Table 1: Difference between Battery Anode and Cathode
| Aspect | Anode | Cathode |
|---|---|---|
| Direction of Current | Current flows into the anode | Current flows out of the cathode |
| Electron Flow | Electrons are released (oxidation) | Electrons are gained (reduction) |
| Charge | Typically considered negative | Usually labelled as positive |
| Chemical Reactions | Site of oxidation reactions | Site of reduction reactions |
| Mass Changes | May undergo changes in mass | Generally, experiences minimal mass changes |
| Material Composition | Often graphite or lithium-based materials | Often metal oxides or lithium-based materials |
| Function in Battery | Supplies electrons to the external circuit during discharge | Accepts electrons from the external circuit during discharge |
| Ion Movement | Attracts positively charged ions (cations) | Attracts negatively charged ions (anions) |
| State of Charge | Typically depleted during discharge | Typically enriched during discharge |
| Potential Difference | Generally, has a lower potential relative to the cathode | Generally, has a higher potential relative to the anode |
| Reactivity | Tends to be more reactive | Tends to be less reactive |
| Conductivity | Typically, higher electrical conductivity | Typically, lower electrical conductivity |
Part 3. Battery positive and negative electrodes
To reduce confusion, engineers often talk about positive and negative electrodes, especially in rechargeable batteries.
Here’s how it works:
- During discharge, the positive electrode is the cathode
- During charging, the positive electrode becomes the anode
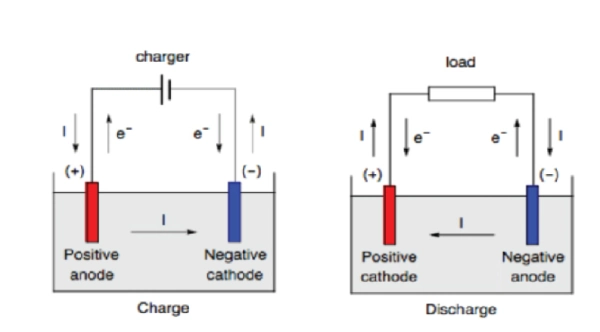
At the Anode, an oxidation reaction occurs: the loss of electrons. A reduction reaction occurs at the cathode, which is a gain of electrons for the electroactive species.
But if we deal with batteries, oxidation and reduction sometimes occur at the same electrode. This can occur during charging or discharging. So, it is important to refer to electrodes with positive or negative electrodes instead of cathode and anode.
1 Is an anode negative or positive?
The positive electrode has a higher potential than the negative electrode. So, when the battery discharges, the cathode acts as a positive, and the anode is negative.
2 Is the cathode negative or positive?
Similarly, during the charging of the battery, the anode is considered a positive electrode. At the same time, the cathode is called a negative electrode.
Part 4. Battery positive vs negative: What’s the difference?
For a better understanding, we summarise the concept of negative and positive electrodes for batteries in the following table.
Table 2: Difference Between the battery positive and negative electrodes
| Aspect | Positive Electrode | Negative Electrode |
|---|---|---|
| Location during Discharge | Cathode | Anode |
| Location during Charging | Anode | Cathode |
| Electrochemical Reaction | Reduction reaction (gain of electrons) | Oxidation reaction (loss of electrons) |
| Charge | Positive | Negative |
| Potential | Higher potential relative to the negative electrode | Lower potential relative to the positive electrode |
| Function in Battery | Supplies electrons to the external circuit during discharge | Accepts electrons from the external circuit during discharge |
| Ion Movement | Attracts positively charged ions (cations) | Attracts negatively charged ions (anions) |
| State of Charge | Enriched during discharge | Depleted during discharge |
| Conductivity | Lower electrical conductivity | Higher electrical conductivity |
Part 5. Lithium ion batteries material

Lithium-ion batteries can flow the right amount of charge. This is why they have flawless components, i.e., Anode and Cathode material. As battery technology emerges, the anode and cathode materials play a significant role. So, let’s discuss the differences between materials used in the anode and cathode of Lithium-Ion batteries.
1 What Is Lithium-ion Battery Cathode?
Lithium-ion cathode stores and releases the lithium ions during the charging and discharging of the battery. It is a positive electrode and undergoes a reduction reaction during discharge. Hence, the lithium-ions are captured within the structure.
2 What Is Lithium-ion Battery Cathode Materials?
When we study the complete working of cathodes, we see that their manufacturing material also plays a crucial role. Because they help estimate the characteristics and overall performance of the battery.
So, here is an overview of some commonly used cathode materials in Lithium-ion Batteries.
- Lithium Cobalt Oxide (LiCoO2) is generally used in commercial Li-Ion batteries. Because they have high energy density and stable cycling performance; however, cobalt is an expensive material and is less available. So, research is being carried out to find alternative materials for cathodes.
- Lithium Iron Phosphate (LiFePO4): Regarding vehicles, they also require batteries. So, LiFePO4 is considered a popular choice in this aspect. The reason for its preference is it is safe and quite stable. Moreover, it has less energy density than Lithium Cobalt Oxide (LiCoO2). But it has a longer cycle of life and better thermal stability.
- Lithium Nickel Manganese Cobalt Oxide (NMC) :It is also quite popular among electric vehicles and portable electronic devices. So, MNC has balanced thermal stability, high energy density, and a long life cycle.
- Lithium Nickel Cobalt Aluminum Oxide (NCA): Just like NMC, NCA is also considered a good fit for high-performance applications i.e., grid storage systems and electric vehicles.
- Layered Oxides (LMO) and Spinel Oxides: This material belongs to the class of layered oxides cathodes. Hence, they have high energy density. Moreover, it has a good rate capability, making it suitable for several applications.
To see a full comparison of different lithium-ion battery types, including NMC, LFP, and LTO, take a look at our guide on NMC vs LFP vs LTO batteries.
3 What Is Lithium-ion Battery Anode?
While the lithium-ion anode is present opposite to the cathode, it has a negative charge. Hence, it undergoes an oxidation reaction during the charging and discharging of the battery.
4 What Is Lithium Battery Anode Materials?
Like cathode materials, Anode materials can be of different types and serve different purposes. These materials are essential for storing and releasing energy during the charging and discharging of the batteries. So here are some commonly used Lithium-Ion anode materials;
- Lithium Alloyed Metals: these alloy metals have high stability and are safe to use. Hence, they are commonly used as anodes. One commercial application of it is lithium titanate (Li4Ti5O12).
- Carbon-Based Materials: The most commonly used material for anode is Graphite. It has a layered structure and smoothly allows lithium ions to intercalate during the charging. Moreover, its capacity for intercalation is limited, which affects its performance.
- Non-Graphitic Carbon Materials: Materials like amorphous and hard carbon are included. So, they have lesser initial capacities than graphite. However, they offer higher reversible capacities and longer life cycle stability.
- Novel Graphite Anodes: Researchers are searching for modified graphite forms, like kish graphite. It has high electrochemical properties. The purpose of the modified forms is to improve the performance and stability of graphite-based anodes.
- Silicon-Based Anodes: Silicon has a high theoretical capacity for lithium storage. So this makes it a suitable option for anode materials. However, as silicon goes through a large volume during the cycling process, the chances of electrode degradation are higher. Nanomaterials are also investigated to overcome this challenge because of their smaller volume.
So, the overall choice of materials for the lithium-ion battery anode and cathode is important to optimize the battery’s performance.
Part 10. FAQs
Does anode vs cathode change in different battery types?
Yes. While the electrochemical definitions stay the same, polarity behavior can differ between primary batteries, rechargeable batteries, and electrochemical cells like fuel cells.
How can you identify the anode and cathode without labels?
You can identify them by observing electron flow or reaction type: oxidation occurs at the anode, and reduction occurs at the cathode, regardless of terminal markings.
Why do textbooks and datasheets sometimes contradict each other?
Because some sources define electrodes based on electrochemistry, while others use terminal polarity during discharge. Without context, this leads to apparent contradictions.
Can incorrect anode–cathode identification damage a battery?
Yes. In lithium-ion systems, incorrect assumptions can lead to improper charging, reduced efficiency, or safety risks such as overheating.
Related Tags:
More Articles

Top 10 3.7V 18650 Battery Recommendations
Top 10 3.7V 18650 battery recommendations with specs, comparisons, applications, and tips to choose the right 18650 rechargeable battery.
Lithium Battery Repair: How to Fix & Restore Your Lithium‑Ion Battery
Learn how to repair lithium-ion batteries safely with practical tips, BMS tricks, and DIY cell balancing for longer battery life.
What Are Lithium Button Batteries?
What lithium button batteries (coin cell batteries) are, key CR types, sizes, voltage, lifespan, safety, and how to choose the right one for electronic devices.
Can You Really Revive a Dead Lithium-Ion Battery?
Many battery fixes are unsafe. Discover proven ways to revive lithium-ion batteries—and the risky tricks you should avoid.
Differences Between Power Battery and Energy Battery
Compare power batteries and energy batteries by performance and applications in EVs, energy storage, and industrial systems.



