Since 1859, rechargeable batteries have been working like a magic box. They are backup power and provide energy to the different gadgets. These batteries can recharge. So, they are the best option for electronic devices, smartphones, and even vehicles. This article will discuss the definition of rechargeable batteries and how they work. We will explain its application and different types as well.
Part 1. What are the rechargeable batteries?
Rechargeable batteries are also called secondary cells. They potentially consist of a reversible cell reaction that helps them to recharge and regain their electric potential through the flow of currents. Compared with primary (not reversible) cells, rechargeable batteries can be charged and discharged numerous times. Moreover, rechargeable batteries have diverse applications, such as electronic devices, smartphones, and electric vehicles.
Part 2. Construction of rechargeable batteries
So, batteries are the collection of one or more cells. The chemical reactions create the flow of electrons in a circuit. Three basic components in the battery are:
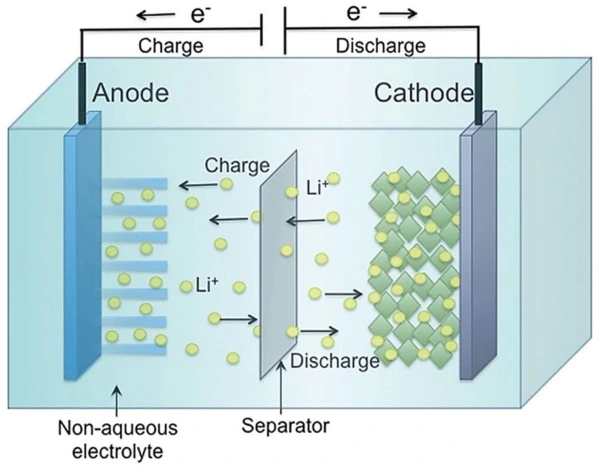
- Anode: Here, oxidation reactions (loss of electrons) take place. Moreover, common anode materials may include graphite, lithium metal, and lithium-ions.
- Cathode: Unlike an anode, the cathode is an electrode where reduction reactions (gain of electrons) occur. Moreover, the material of the cathode depends on the type of battery. You can use different lithium alloys here.
- Electrolyte: The electrolyte is a substance that chemically reacts with anode and cathode in a battery. It is a medium that allows the flow of ions inside the battery. The anode and cathode are immersed in it.
- Separator: This is a porous membrane. It separates the anode and cathode to avoid short-circuiting. Moreover, it is chemically and electrically stable. The most used separators are Polyethylene or polypropylene membranes.
Part 3. How do rechargeable batteries work
So, let’s discuss how rechargeable batteries work. A battery is a container with two compartments—one compartment with a surplus of electrons and the other with an electron deficit. Negative charges present in the first compartment repel each other. They are pushed through a device and wind up in the second compartment.
Moreover, it converts chemical energy into electricity. When the battery is hooked up, and electrons are allowed to move, a chemical reaction starts in the battery. It causes the release of surplus electrons.
Chemistry of an anode, electrolyte, and cathode
Chemical reactions build up electrons at the anode. It creates the electrical difference between the anode and cathode. This difference is due to an unstable build-up of electrons. They want to rearrange and get rid of this difference. They will repel each other and move towards the cathode. The electrolyte keeps moving straight from the anode to the cathode within the battery. This flow transforms chemical energy into an electrical form that powers any device or machine attached to it, e.g., a car and battery.
Part 4. Rechargeable vs non-rechargeable batteries
Both rechargeable and non-rechargeable batteries serve as backup power in different applications. But they have distinct characteristics. Both have different working and composition. So, here is a list of differences between rechargeable and non-rechargeable batteries. They will help us understand how rechargeable batteries work.
| Feature | Rechargeable Batteries | Non-Rechargeable Batteries |
|---|---|---|
| Ability to Recharge | Can be recharged multiple times | Cannot be recharged |
| Electrode Materials | Often use lithium-ion or NiMH | Often use alkaline or zinc-carbon |
| Initial Cost | Generally higher upfront cost | Lower upfront cost |
| Long-Term Cost | May be more cost-effective over time due to the reuse | May require frequent replacement, and can be more expensive over time |
| Environmental Impact | Can reduce waste from disposable batteries | They should recycle. |
| Energy Density | Typically, lower energy density | Typically, higher energy density |
| Self-Discharge Rate | Generally higher self-discharge rate | Generally lower self-discharge rate |
| Voltage Stability | Voltage tends to remain stable over the discharge cycle | Voltage may drop gradually over the discharge cycle |
| Usage in High-Drain Devices | Maybe less suitable for high-drain devices due to lower discharge rates | Often suitable for high-drain devices due to higher discharge rates |
Part 5. Types of rechargeable batteries
Four main types of rechargeable batteries are:
1.Nickel-Cadmium (NiCd) batteries
The NiCd batteries have positive and negative electrodes. It uses nickel oxide hydroxide (NiOOH) as a positive (cathode) and cadmium (Cd) as a negative (anode). It uses potassium hydroxide (KOH) as an electrolyte. So, this battery is relatively cheaper. Moreover, it can allow high currents. It is a suitable option for power tools and industrial applications. It works well in cold temperatures.
2. Nickel-Metal Hydride (NiMH)
NiMH batteries use nickel oxyhydroxide (NiOOH) on the cathode (positive electrode) while hydrogen-absorbing alloy at the anode (negative electrode. Like nickel-cadmium batteries, potassium hydroxide (KOH) or sodium hydroxide (NaOH) is an electrolyte. So, this battery offers high energy density. Moreover, it is eco-friendly.
3. Lithium-ion (Li-ion) Batteries
Li-ion batteries use lithium cobalt oxide (LiCoO2) or lithium iron phosphate (LiFePO4) at the cathode (+ve charge). On the other hand, it uses graphite at anode (-ve charge). Moreover, if we talk about its electrolytic solution, it mainly comprises lithium salt dissolved in an organic solvent. Li-ion batteries have high energy density. They are quite lighter in weight and have a low discharge rate. So, it allows partial charging and discharging without affecting the performance.
4. Lithium-ion polymer (LiPo) Batteries
The composition of LiPo batteries is similar to Li-ion batteries, but they use a solid polymer electrolyte. They have low energy density and are more expensive to produce than Li-ion batteries.
Part 6. How long do rechargeable batteries last?
Different batteries have different lifespans. This depends on serval factors. So, here are some average lifespans of different batteries.
- Lithium-ion (Li-ion) Batteries – 300 to 500 charge-discharge cycles
- Nickel Metal Hydride (NiMH) Batteries – 500 to 1000 charge-discharge cycles
- Lead-Acid Batteries – 3 to 5 years.
- Lithium Iron Phosphate (LiFePO4) Batteries – 2000 to 5000 charge-discharge cycles or more
Part 7. Factors affecting battery life
So, here are some factors that affect the battery life, which are mentioned below.
- Temperature: Temperature directly affects the battery life. Moreover, we can say that the battery performance varies with temperature variation. So, higher temperature charging and discharging will affect the negative electrode performance and eventually deteriorate, leading to battery failure.
- Charge cycles: The charge-discharge cycle also has an impact on battery life. Each cycle tends to contribute to the wear and tear of battery components. Moreover, it reduces the capacity and ultimately decreases the lifespan.
- Battery age: With time, the battery capacity decreases. So, the older your battery is, the less charge it will hold. This results in shorter battery life. Moreover, its efficiency will be reduced, and it will have a shorter life cycle due to self-discharging.
Part 8. Maintenance of rechargeable batteries
There are some precautionary steps to maintain the performance of rechargeable batteries:
- Don’t charge the battery until its battery percentage is down to 20%.
- Avoid keeping it plugged in at 100%.
- Don’t let it get too hot.
- Avoid upgrading it when the batteries die.
Thus, rechargeable batteries are more cost-effective, better for the environment, and more stable than alkaline batteries.
Part 9. Pros and cons of rechargeable batteries
Convert the following table into the form of HTML table code Pros Cons Can be recharged multiple times Higher upfront cost Cost-effective over the long term Lower energy density than non-rechargeable batteries Reduce waste from disposable batteries Limited lifespan (finite number of charge cycles) Convenient for frequent use Self-discharge rate may be higher than non-rechargeable batteries Environmentally friendly when recycled properly Performance may degrade over time Available in various chemistries for different applications May not be suitable for high-drain devices Generally safer than non-rechargeable batteries Longer charging time compared to disposable batteries.
Part 10. Applications of rechargeable batteries
So, after getting deep knowledge of how rechargeable batteries work, here are some applications of rechargeable batteries mentioned below.
- These batteries can be used to power portable electronic devices like cameras, power tools, and appliances,
- Rechargeable batteries can be used for electricity generation distribution and in-stand-alone power systems.
- They can be used to power electric vehicles ranging from scooters to locomotives.
Part 11. Challenges of rechargeable batteries
Batteries play a significant role in the backup power of vehicles. They have various applications in different industries. But every rose comes with thrones. So, it has some limitations, and some problems can occur with rechargeable batteries. So, these problems are mentioned below.
1. Extreme Temperatures
Extreme temperature is a major problem as it affects the performance and safety of a battery. Suppose the battery temperature becomes too high during charging and discharging. In that case, it will decrease performance, safety risks, and even explosions.
2. Charger issues
Charger issues include a dead battery, wiring issues, or any improper placement on the charger. Rechargeable batteries can be sensitive to the chargers or charging methods. Because undercharging, overcharging, or charging at a high voltage leads to battery degradation. These issues lead to a reduction of capacity. Moreover, the chances of safety hazards, such as overheating or explosion, increase. So, using specific chargers and charging the batteries to their limit is important.
3. Battery age and explosion
Rechargeable batteries degrade with time. It is generally because of chemical reactions and usage cycles. Over time, the batteries also become more prone to internal shorts. Moreover, the risk of overheating and potentially exploding also increases with time. So, it can be reduced if you take good care of your battery. Avoid overcharging, and don’t let your battery completely discharge. It can also lead to an explosion.
4. Corrosion issues
Corrosion can reduce the battery’s capacity. Moreover, it increases internal resistance and potential hazards. It can occur because of several factors, such as exposure to moisture, high temperatures, or incompatible materials within the battery. You can fix it by using proper storage. You can avoid battery exposure to harsh environments.
Part 12. Conclusion
In conclusion of how do rechargeable batteries work, we can say that rechargeable batteries or secondary cells work due to reversible reactions. They comprise anode, cathode, casing, separator, and charge collector. Moreover, they work on the principle of conversion of energies, such as electrical energy, into chemical energy and vice versa. They generate electricity when they allow the electrons to flow from the anode to the cathode. We can store this energy and give it to some external source. So, you just need to attract the source with the battery terminals for the latter one.
Related Tags:
More Articles

Comprehensive Guide to Choosing the Right Cart Battery
Choosing the right cart battery ensures optimal performance and longevity. This guide covers cart battery types and helps you make an informed choice.
The Ultimate Guide to 18650 Button Top Battery
18650 button top batteries are popular for their high energy density and reliability. This guide covers their key features, usage, and maintenance tips.
The Power of Slim: Unveiling the Potential of Flat Lithium Ion Battery
Flat lithium-ion batteries power devices from phones to vehicles. This article explores their design, benefits, types, applications, charging, and safety.
The Comprehensive Guide to Battery Balancing and Battery Balancer
Battery balancing and balancers optimize performance, longevity, and safety. This guide covers techniques and tips for choosing the right balancer.
10 Key Facts About Drone Battery for 2024
Uncover crucial insights with "10 Key Facts About Drone Battery for 2024." Learn the latest trends and essential details on drone batteries.