Batteries are ubiquitous today, powering everything from smartphones to electric vehicles. We must familiarize ourselves with the common battery terminology to better understand these powerhouses. This comprehensive guide will explore the various types of batteries, their components, performance metrics, charging and discharging processes, battery connections, and safety and maintenance considerations. Let’s start!
Part 1. Battery types
What are the main types of batteries? Here’s a quick overview:
- Lead-Acid Battery: Reliable, used in vehicles and UPS systems.
- Lithium-Ion Battery: Lightweight, high energy density, ideal for electronics and EVs.
- Nickel-Cadmium Battery (NiCd): Durable, performs well in extreme conditions.
- Nickel-Metal Hydride Battery (NiMH): Eco-friendly, great for hybrid vehicles and gadgets.
- Alkaline Battery: Long-lasting, perfect for everyday devices.
- Zinc-Carbon Battery: Affordable, suitable for low-power use.
Part 2. Battery components
To understand how batteries work, let’s explore their key components:
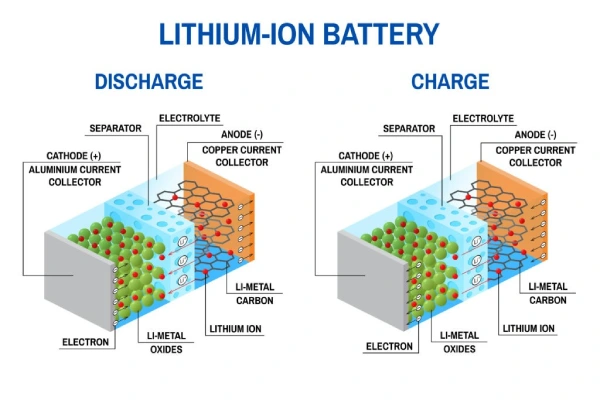
Electrodes
Batteries consist of two electrodes: the anode and the cathode. The anode is the negative electrode, where oxidation occurs during discharge. At the same time, the cathode is the positive electrode, where reduction takes place.
Electrolyte
The electrolyte acts as a medium that allows the movement of ions between the electrodes. It facilitates the chemical reactions necessary for energy storage and release within the battery.
Anode
The anode is the electrode where oxidation occurs during the battery’s discharge cycle. It releases electrons and ions into the electrolyte, generating the flow of electric current.
Cathode
The cathode is the electrode where reduction takes place during discharge. It accepts the electrons and ions from the electrolyte, completing the electric circuit.
Separator
The separator is a porous material placed between the anode and cathode to prevent electrical short circuits while allowing the passage of ions.
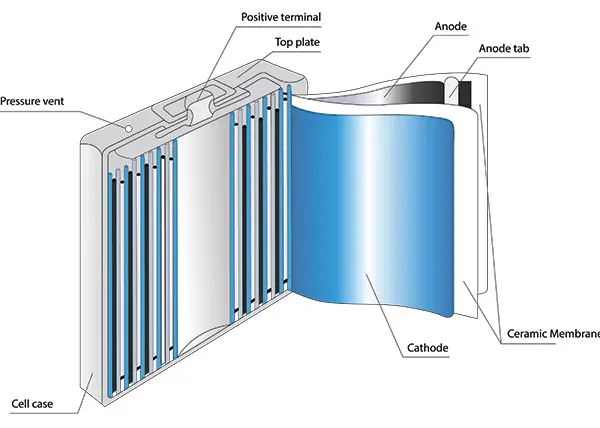
Terminal
Terminals are the external connection points of a battery, allowing for the transfer of electrical current to external devices or charging sources.
Casing
The casing houses and protects the battery’s internal components, providing structural integrity and insulation.
Cell
A cell refers to the basic unit of a battery. It consists of electrodes, an electrolyte, and a separator. Multiple cells can be connected to form a higher voltage or capacity battery.
Part 3. Battery performance metrics
Several vital metrics are crucial for evaluating battery performance:
Capacity
Capacity represents the amount of electrical energy a battery can store and deliver. Typically, we measure it in ampere-hours (Ah) or milliampere-hours (mAh). Higher capacity batteries provide more extended operating times.
Voltage
Voltage is the electrical potential difference between the battery’s positive and negative terminals. It determines the force at which electrons flow through a circuit. We commonly measure battery voltage in volts (V).
Current
Current denotes the flow of electric charge in a circuit and is measured in amperes (A). It represents the rate at which electrons move through the circuit.
Energy Density
Energy density indicates the amount of energy a battery can store per unit of its volume or mass. We express it in watt-hours per liter (Wh/L) or kilogram (Wh/kg). Batteries with higher energy density offer more excellent energy storage capabilities.
Power Density
Power density refers to the amount of power a battery can deliver per unit of its volume or mass. We measure it in watts per liter (W/L) or kilogram (W/kg). Batteries with higher power density can supply more power in a shorter period.
Cycle Life
Cycle life refers to the number of charge and discharge cycles a battery can undergo before its capacity significantly degrades. A longer cycle life indicates better durability and longevity.
Self-Discharge Rate
Self-discharge rate represents the rate at which a battery loses its charge over time when unused. Batteries with low self-discharge rates retain their charge for extended periods, ensuring they are ready for use when needed.
Efficiency
Efficiency measures how effectively a battery converts stored energy into usable electrical energy. Higher efficiency means less energy loss during charging and discharging processes.
Internal Resistance
Internal resistance characterizes the opposition to the flow of electrical current within a battery. Batteries with low internal resistance can deliver currents more efficiently.
C-Rating
The C-rating indicates a battery’s discharge rate relative to its capacity. For example, a battery with a 1C rating can discharge its entire capacity in one hour. Higher C-ratings allow for faster discharge rates.
Amps (Amperes)
The unit of electric current represents the rate of flow of electric charge. It measures the current flow through a circuit at a given moment.
Amp-hour
A unit of electric charge represents the amount of electrical energy transferred by a current of one ampere flowing for one hour. People commonly use it to measure the capacity of a battery to store energy.
The unit of power represents the rate at which work or energy transfers. You calculate it by multiplying voltage by current.
Watt-hour
This unit of energy represents the energy consumed or produced by a device with a power rating of one watt operating for one hour. People commonly use it to measure a battery’s energy capacity or an electrical device’s energy consumption over time.
Part 4. Charging and discharging
Understanding the charging and discharging processes is essential for maintaining battery performance:
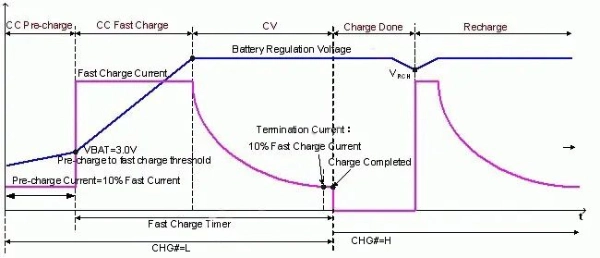
Charging
Charging involves supplying electrical energy to a battery to restore its capacity. Proper charging techniques and voltage levels are crucial to ensure battery longevity and safety.
Discharging refers to releasing stored electrical energy from a battery to power external devices. The discharge rate should be within the battery’s specified limits to maintain optimal performance.
Overcharging
Overcharging occurs when someone charges a battery beyond its recommended capacity or voltage. This can lead to reduced battery life, increased heat generation, and even safety hazards.
Deep Discharge
Deep discharge occurs when someone discharges a battery to deficient voltage levels. This can cause irreversible damage, reducing the battery’s capacity and lifespan.
Trickle Charging
Trickle charging is a low-current method used to maintain batteries at total capacity during extended periods of inactivity or storage. It prevents self-discharge and keeps the battery ready for use.
Fast Charging
Fast charging techniques allow rapid replenishment of a battery’s capacity, significantly reducing charging times. However, if not implemented correctly, fast charging may generate more heat and impact long-term battery health.
Float Charging
Float charging involves maintaining a battery at a constant voltage level, typically lower than its full charge voltage. People commonly use it in applications where batteries need to be constantly available, such as emergency backup systems.
Battery Management System (BMS)
A battery management system is an electronic system that monitors and controls various aspects of battery operation, including charging, discharging, and temperature regulation. It ensures optimal performance, safety, and longevity of the battery.
Part 5. Battery connections
Battery connections play a crucial role in achieving desired voltage and capacity levels:
Serial Connection
Serial or series connections involve connecting batteries in a chain-like configuration. The positive terminal of one battery is connected to the negative terminal of the next battery, increasing the overall voltage while keeping the capacity unchanged.
Parallel Connection
Parallel connection involves connecting batteries side by side, with their positive and negative terminals connected. This configuration increases the overall capacity while maintaining the voltage level.
Series-Parallel Connection
Series-parallel connection combines both series and parallel connections to achieve the desired voltage and capacity levels. It involves connecting multiple sets of batteries in series and then in parallel.
Part 6. Safety and maintenance
Ensuring safety and proper maintenance practices are essential for prolonging battery life and preventing accidents:
Thermal Runaway
Thermal runaway is when a battery’s temperature rapidly increases due to internal reactions or external factors. It can lead to a self-sustaining, uncontrollable increase in temperature, potentially resulting in a fire or explosion. Batteries are equipped with safety mechanisms such as thermal sensors, protection circuits, and temperature management systems to prevent thermal runaway.
Overheating
Overheating is when a battery operates at a temperature higher than its recommended range. High temperatures can accelerate chemical reactions within the battery, leading to reduced capacity, shorter lifespan, and increased risk of thermal runaway. Adequate cooling and proper ventilation are essential to prevent overheating and maintain battery performance and safety.
Short Circuit
A short circuit occurs when a battery’s positive and negative terminals are connected directly, bypassing the intended circuitry. This can lead to a sudden discharge of a large amount of current, potentially causing damage to the battery and surrounding components. Proper insulation, protection circuits, and handling precautions are necessary to prevent short circuits.
Venting
Some batteries, especially lithium-ion batteries, have safety features such as pressure relief valves or vents. These features allow the release of gases or built-up pressure in case of overcharging or other potentially hazardous conditions, ensuring the safety of the battery and its surroundings.
Battery Degradation
Battery degradation refers to the gradual loss of a battery’s capacity and performance over time. Factors such as usage patterns, temperature exposure, and charging/discharging cycles can contribute to degradation. Regular maintenance, proper charging practices, and avoiding extreme operating conditions can help minimize degradation and extend the battery’s lifespan.
Cell Balancing
Cell balancing is a process used in multi-cell batteries, such as lithium-ion battery packs, to ensure each cell has a balanced state of charge. Balancing prevents overcharging or over-discharging specific cells, leading to capacity imbalances and reduced overall battery performance. One can achieve balancing by actively monitoring and controlling cell voltages during charging and discharging.
State of Charge (SoC)
The state of charge refers to the remaining capacity in a battery, expressed as a percentage. Monitoring the SoC helps prevent over-discharging, which can damage the battery, and ensures the battery’s availability for use. Some batteries have built-in SoC indicators, while others require external measurements or sophisticated algorithms to estimate the SoC accurately.
State of Health (SoH)
The state of health refers to a battery’s overall condition and performance capability compared to its original specifications. It considers capacity loss, internal resistance increase, and overall degradation. Assessing the SoH helps determine the remaining lifespan and reliability of the battery, allowing for timely maintenance or replacement if necessary.
Part 7. FAQs
-
Which battery type is the most efficient?
Lithium-ion batteries are often considered the most efficient due to their high energy density, long cycle life, and lightweight design. -
How can I extend the lifespan of my battery?
To prolong battery life, avoid overcharging or deep discharging, store at room temperature, and use a proper Battery Management System (BMS). -
What is the difference between capacity and energy density?
Capacity is the total energy a battery can store, while energy density measures how much energy is stored per unit of volume or weight. -
Can non-rechargeable batteries be recycled?
Yes, non-rechargeable batteries can be recycled. Many recycling centers accept them to recover valuable materials like zinc and manganese.
Related Tags:
More Articles

What Is a Disk Battery? A Simple Guide for Non-Tech Users
A disk battery is a small, round cell used in watches, remotes, and other electronic devices. It delivers steady power for compact, low-drain devices.
What Battery Powers a Space Heater?
Discover the type of battery that powers space heaters and learn how to choose the right one for efficient heating in your home or office.
What Is an LR14 Battery? Learn About This C-Size Cell
The LR14 battery, also known as a C battery, delivers steady power. Learn its specs, uses, lifespan, and how it compares to other battery types.
Watch Battery Dimensions Chart: Sizes, Voltages, and Equivalents Explained
Understanding watch battery dimensions helps you choose the right size, voltage, and equivalent model to keep your watch running safely and smoothly.
How Long Can You Rely on Battery-Powered Generators?
Discover battery generator runtime & lifespan factors. Learn how to maximize performance and choose the right power solution.