- Part 1. Historical evolution: the path to alkaline
- Part 2. Limitations of early batteries
- Part 3. Breakthroughs that paved the way
- Part 4. Major innovators and the companies that made it happen
- Part 5. The production process: from raw material to power source
- Part 6. The Chemistry behind alkaline batteries
- Part 7. Unique features and benefits
- Part 8. Alkaline battery series and types
- Part 9. Recycling: giving batteries a second life
- Part 10. Final thoughts
- Part 11. FAQs
It’s hard to imagine life without batteries. From remote controls to flashlights, toys to clocks, and wireless mice to digital cameras, batteries are deeply embedded in modern living. But among all the types, one stands out for its reliability, efficiency, and history: the alkaline battery. The story behind the alkaline battery invention isn’t just about chemistry; it’s a tale of curiosity, perseverance, and game-changing innovation that has powered generations.
Part 1. Historical evolution: the path to alkaline
Before the alkaline battery came into being, portable energy was a frustrating experience. In the 1800s, Alessandro Volta gave us the voltaic pile—the world’s first battery. While revolutionary, it was bulky and unstable. Over the next century, inventors explored ways to store energy more safely and compactly.
By the early 20th century, zinc-carbon batteries had become the standard. These dry cells were affordable and widely used. However, they had several limitations: they drained quickly, leaked frequently, and degraded rapidly when exposed to varying temperatures. As household electronics became more advanced, these batteries simply couldn’t keep up.
The push for a better solution was on.
Part 2. Limitations of early batteries
To understand why the alkaline battery was such a breakthrough, it’s important to recognize what came before it.
Early zinc-carbon batteries relied on an acidic electrolyte, typically ammonium chloride or zinc chloride. These batteries had high internal resistance, which meant that voltage dropped quickly under load. In other words, when you tried to power something that required steady energy, the battery would struggle.
Other key issues included:
- Leakage: The acidic electrolyte often corroded the casing.
- Low shelf life: Batteries deteriorated even when not in use.
- Limited capacity: Couldn’t power high-drain devices for long.
Understanding Battery Leaks: Why They Happen and How to Stay Safe
By mid-century, the need for a longer-lasting, safer, and more powerful battery was urgent. Engineers across the globe searched for alternatives. The answer lay in a basic chemical shift.
Part 3. Breakthroughs that paved the way
The first real breakthrough came with the realization that alkaline electrolytes could drastically improve battery performance. Unlike their acidic counterparts, alkaline materials allowed for:
- Lower internal resistance
- Higher current output
- Longer shelf life
The key was potassium hydroxide (KOH), a strong base that offered excellent ionic conductivity. Combining this with zinc (as the anode) and manganese dioxide (as the cathode) laid the foundation for the modern alkaline cell.
But chemistry alone wasn’t enough. The engineering challenge was to make the battery stable, safe, and cost-effective for mass production.
Enter Lewis Urry.
Part 4. Major innovators and the companies that made it happen
Lewis Urry: The Father of the Alkaline Battery
In 1955, Canadian-born engineer Lewis Urry was working at the Eveready Battery Company (now known as Energizer). Tasked with extending battery life, Urry and his colleagues experimented with materials. He noticed that powdered zinc provided a greater reactive surface area than solid zinc sheets, enabling higher efficiency.
After months of trial and error, he created a prototype alkaline battery using zinc powder, manganese dioxide, and potassium hydroxide. The result? A battery that lasted several times longer than zinc-carbon cells.
The Companies Behind the Revolution
- Eveready (Energizer): Commercialized Urry’s invention in 1959, launching a new era in portable power.
- Duracell: Another early adopter, known for consistent innovation and marketing.
- Panasonic, Sony, and Toshiba: These Japanese giants refined manufacturing processes and expanded alkaline batteries worldwide.
Thanks to these innovators, alkaline batteries became mainstream in homes, industries, and military applications.
Part 5. The production process: from raw material to power source
Producing an alkaline battery is a sophisticated, multi-step process. Precision is everything.
Step-by-step Overview:
- Material Preparation: Zinc powder, manganese dioxide, and electrolytes are precisely measured.
- Cathode Assembly: Manganese dioxide is compacted into the battery casing.
- Anode Formation: Zinc paste is inserted as the anode.
- Electrolyte Addition: Potassium hydroxide is introduced as the conductive medium.
- Separator Installation: A non-woven fabric separates anode and cathode to prevent short circuits.
- Sealing: The battery is sealed with a metal cap and insulator.
- Testing & Quality Control: Each batch is checked for voltage, leaks, and longevity.
Part 6. The Chemistry behind alkaline batteries
Alkaline batteries operate through an electrochemical reaction. Let’s break it down.
- Anode (Negative Electrode): Zinc (Zn)
- Cathode (Positive Electrode): Manganese dioxide (MnO2)
- Electrolyte: Potassium hydroxide (KOH)
The Reaction:
At the anode, zinc oxidizes: Zn → Zn2+ + 2e⁻
At the cathode, manganese dioxide is reduced: 2MnO2 + 2H2O + 2e⁻ → 2MnOOH + 2OH⁻
These reactions create a flow of electrons—electricity. The alkaline environment stabilizes the reaction, ensuring steady output until the reactants are used up.
This chemistry provides higher energy density, lower self-discharge, and better performance under load compared to acidic systems.
Part 7. Unique features and benefits
Why have alkaline batteries stood the test of time? Because they offer an outstanding balance of performance, cost, and convenience.
Key Features:
- High Energy Density: More power in a compact form.
- Long Shelf Life: Can be stored for 5–10 years.
- Leak Resistance: Modern seals prevent corrosive leaks.
- Stable Voltage: Delivers consistent output.
- Temperature Tolerance: Works from -20°C to 54°C.
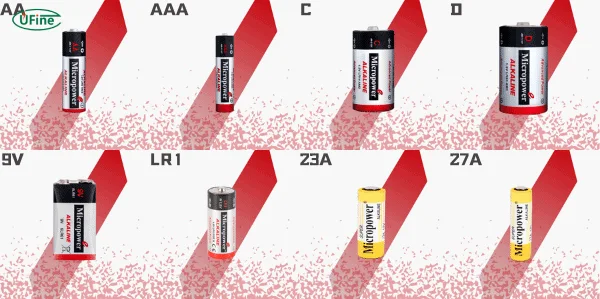
Part 8. Alkaline battery series and types
Alkaline batteries come in various sizes and capacities. Each is suited for specific applications.
| Type | Code | Common Uses |
|---|---|---|
| AA | LR6 | Remotes, toys, clocks |
| AAA | LR03 | Flashlights, toothbrushes |
| C | LR14 | Radios, musical toys |
| D | LR20 | Boom boxes, high-drain devices |
| 9V | 6LR61 | Smoke alarms, detectors |
| Button Cells | LR44, LR1130 | Watches, hearing aids, calculators |
The diversity of formats ensures that alkaline technology supports a wide range of electronic products.
Part 9. Recycling: giving batteries a second life
With billions of alkaline batteries used annually, recycling is vital.
Why Recycle?
- Prevent Landfill Overflow: Batteries take decades to degrade.
- Recover Materials: Zinc, manganese, and steel are reusable.
- Protect Environment: Prevents soil and water contamination.
How to Recycle:
- Drop off at designated collection bins.
- Use municipal hazardous waste centers.
- Check manufacturer take-back programs.
While alkaline batteries contain less toxic material than older types, proper disposal is still essential for environmental health.
Part 10. Final thoughts
The alkaline battery invention didn’t just improve energy storage—it transformed how we live.
Even in the age of lithium-ion and wireless charging, the humble alkaline battery remains indispensable. Why? Because it’s reliable, accessible, and trusted. And sometimes, the simplest solution really is the most powerful.
Part 11. FAQs
Who invented the alkaline battery?
Lewis Urry, in 1959, while working for Eveready.
What makes a battery “alkaline”?
It uses an alkaline electrolyte—usually potassium hydroxide—instead of an acidic one.
Are alkaline batteries rechargeable?
Standard ones are not. However, there are specialized rechargeable alkaline batteries.
How long do they last?
They can power devices for 2–5 times longer than zinc-carbon batteries.
Can I recycle them?
Yes. Many recycling centers accept alkaline batteries.
Related Tags:
More Articles
How to Revive Dead Batteries and Fix Lithium Batteries that Won’t Charge?
Learn how to safely revive a dead lithium-ion battery, troubleshoot charging issues, fix lithium battery problems, and extend battery life.
LiPo Battery Charge Rate Calculator
Calculate safe LiPo, Li-ion, and LiFePO4 battery charging times and rates. Prevent overcharging, extend life, and optimize performance.
4.0Ah vs. 2.0Ah Battery: Key Differences and Which is Best for Your Projects
Compare 2Ah vs 4Ah batteries: meaning, runtime, weight, and best tool uses. Learn if 4.0Ah is worth it for your cordless tools and DIY projects.
How Long do Lithium Batteries Last?
Learn how long lithium batteries last, their life expectancy, cycle life, and tips to extend lithium-ion battery lifespan effectively.
Lithium Battery Temperature Range: Everything You Need to Know
Learn optimal lithium battery temperature ranges for use and storage. Understand effects on performance, efficiency, lifespan, and safety.