Have you ever noticed your phone’s battery not lasting as long as it used to? Or maybe your laptop needs to be plugged in more often? This gradual decline in battery performance is a common issue known as battery aging. In this article, we’ll dive into what battery aging is, how it happens, the signs that indicate your battery is aging, factors that can speed up the process, and ways to slow it down. Finally, we’ll address whether it’s still safe and practical to use an aged battery.

Part 1. What is battery aging?
Battery aging is the process where a battery’s ability to hold and deliver charge diminishes over time. This is a natural phenomenon that occurs due to chemical changes within the battery. As a battery ages, you might notice it doesn’t last as long on a single charge, charges more slowly, and may even show physical signs of wear. All batteries age eventually, but understanding how and why it happens can help you manage and mitigate its effects.
Part 2. How do lithium batteries age?
Lithium batteries age through a series of complex chemical reactions. Every time you charge and discharge a lithium battery, it undergoes a process where lithium ions move between the positive and negative electrodes. Over time, these movements cause wear and tear on the battery’s components. The electrolyte solution can degrade, and the electrodes can become less effective. High temperatures and excessive charging cycles can accelerate this degradation. Essentially, each charge cycle contributes a small amount to the overall aging of the battery.
For a single cell, cell aging increases the internal resistance of the battery, and the heat generated during use will also increase. After the battery ages, if the original charging and discharging system is followed, the battery will be overcharged, over-discharged, and over-powered.
For the battery pack: there are differences in the aging speed of the single cells, resulting in greater inconsistency in the battery pack. This will increase the difficulty of BMS management and increase the risk of battery use.
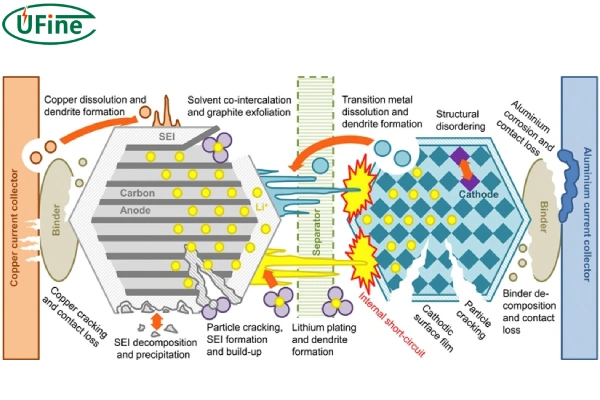
We can see the battery aging from various materials.
1. The negative electrode aging
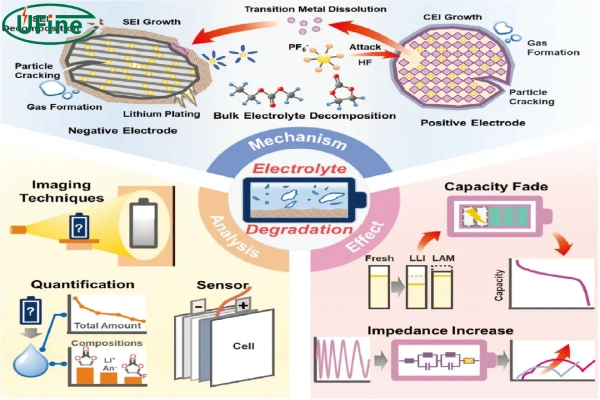
For the negative electrode material the negative electrode material will expand and shrink in volume during the charging and discharging process. During the volume change, the stress generated will cause the negative electrode material to be damaged and ruptured or to be separated from the current collector and freed into the electrolyte.
For the SEI film on the surface of the negative electrode, the SEI film continues to grow during the use of the battery, and the active lithium content is continuously consumed when the SEI film ruptures; a new film layer will form at the rupture, resulting in the irregular overall structure of the SEI film.
After the negative end ages, lithium precipitation and battery capacity decay will occur.
2. The positive electrode aging
The positive electrode material will also expand and shrink in volume during the charging and discharging process. Compared with negative electrode materials, positive electrode materials are more affected by stress. Their structure will gradually collapse, making it impossible for lithium ions to be embedded.
After the positive electrode ages, the capacity will decay.
3. The electrolyte aging
The electrolyte will undergo certain oxidation or decomposition reactions as the number of cycles increases, which will weaken its mass transfer capacity and cause the internal resistance of the battery to increase. The organic solvent in the electrolyte will undergo ester exchange and polymerization reactions during the operation of the battery, and conductive salts such as LiPF6 will degrade in the reaction to form organic phosphates and fluorides.
After the electrolyte ages, the internal resistance of the battery increases.
4. The diaphragm aging
On the one hand, the decomposition products and active materials of the electrolyte block the diaphragm pores. On the other hand, high temperature or circulation causes structural degradation, and there is also the case where the precipitated lithium metal punctures the diaphragm.
After the diaphragm ages, the internal resistance of the battery increases.
5. Aging of the current collector
The aging of the current collector is manifested as corrosion of the metal foil.
Copper is easily oxidized at high potential. Under extreme conditions such as over-discharge, for example, when the voltage is as high as 1.5V, copper will be oxidized into copper ions in the electrolyte, causing the copper current collector to dissolve. The copper ions oxidized by over-discharge will precipitate as metallic copper and deposit on the surface of the negative electrode material during the subsequent charging process. The copper deposited on the surface of the negative electrode will hinder the lithium insertion and removal of the negative electrode and cause the SEI film to thicken.
In an electrolyte with lithium hexafluorophosphate as the electrolyte, a small amount of water can promote the decomposition of the electrolyte and produce stable inorganic salts, thereby inhibiting the corrosion of the aluminum current collector. However, as water is generated, the oxidative decomposition products of the electrolyte undergo electrochemical reactions on the surface of the aluminum foil, causing and accelerating the corrosion of the aluminum foil.
Part 3. Lithium battery aging signs
Recognizing the signs of aging in lithium batteries can help you address issues before they become severe. Here are common signs that a lithium battery is aging:
- Reduced Capacity: The battery doesn’t last as long on a full charge as it used to.
- Longer Charging Times: It takes more time to reach a full charge.
- Inconsistent Battery Levels: The battery percentage might drop suddenly or fluctuate.
- Physical Changes: The battery may swell or show signs of physical damage.
- Increased Heat: The battery might get hotter during use or charging.
- Voltage Drop: The battery’s voltage decreases, leading to poorer performance.
- Unexpected Shutdowns: The device powered by the battery shuts down unexpectedly, even when the battery shows a charge.
Part 4. What factors control the degree of battery aging?
1. Temperature
At low temperatures, the electrochemical reaction rate slows down. During the discharge process, the polarization voltage will increase, and lithium will easily precipitate at the negative end, threatening the diaphragm.
At high temperatures, the electrochemical reaction rate accelerates. The side reactions inside the battery accelerate. Excessive oxidation and decomposition of the electrolyte promote the growth of the SEI film.
During the operation of lithium-ion batteries, due to the low thermal conductivity of its internal components such as electrodes and diaphragms. A temperature gradient will be generated inside the battery cell. The temperature gradient phenomenon is more obvious at high rates and low temperatures. This difference in spatial temperature distribution may aggravate the non-uniform distribution of current density, thereby accelerating battery aging.
2. Charge and discharge rate
For small-capacity batteries, high charge and discharge rates will cause more frequent overcharging and over-discharging, thereby accelerating the aging of small-capacity batteries. The mechanism is mainly the loss of positive electrode active materials caused by diffusion-induced stress generated during high-rate charge and discharge.
3. Overcharge
Overcharge of the negative electrode leads to lithium deposition, overcharge of the positive electrode leads to gas production, and overcharge of the electrolyte leads to aggravated side reactions.
4. Internal inconsistency of the battery
Battery inconsistency is mainly caused by subtle differences in battery manufacturing processes and materials. And the difference in the use environment during the subsequent use of the battery.
The inconsistency is mainly reflected in the battery’s voltage, internal resistance and capacity, and other parameters.
The impact of voltage inconsistency on life is mainly reflected at the end of discharge. Cells with lower voltages will reach the cutoff voltage earlier and reach a completely empty state. At this time, the voltage of other batteries is higher than the cutoff voltage, and there is still a certain capacity inside.
The discharge of the battery at a low SOC state has a greater impact on the battery life, so the aging rate of completely empty cells will be faster than other batteries.
5. Depth of charge and discharge
During the charge and discharge process of lithium-ion batteries, deep charge, and discharge will accelerate the capacity decay of lithium-ion batteries. At this time, the ohmic internal resistance and polarization internal resistance of lithium-ion batteries will increase.
On the other hand, when the depth of charge and discharge is the same, lithium-ion batteries that cycle in the high SOC range are more prone to aging than those that cycle in the low SOC range. This process may be caused by the problem of lithium precipitation in the high SOC range.
In addition, during the accelerated cycle aging of lithium-ion batteries, the aging rate of the battery under constant current charging conditions is higher than the aging rate under constant current and constant voltage charging conditions. Therefore, extending the shelf time during charging and discharging or using a very small current to charge at the end of charging is conducive to extending the battery life.
6. Charging
Pulse charging has a significant effect on battery aging compared to classic CC charging or CC-CV charging. The internal resistance of lithium-ion batteries under pulse charging conditions increases significantly, and the loss of negative electrode active materials is more serious.
Part 5. What will accelerate the li ion battery aging?
Several factors can speed up the aging process of lithium batteries:
- High Temperatures: Heat accelerates chemical reactions inside the battery, leading to faster degradation.
- Overcharging: Continuously charging the battery after it reaches 100% can stress the battery.
- Deep Discharges: Regularly letting the battery run down to zero before recharging can wear it out faster.
- Frequent Charge Cycles: The more often you charge and discharge the battery, the quicker it ages.
- Using Poor Quality Chargers: Chargers that are not compatible or of poor quality can be inefficient and harmful to the battery.
- Physical Damage: Dropping or mishandling the battery can cause internal damage that accelerates aging.
- Poor Storage Conditions: Storing the battery in extreme temperatures or at full charge can reduce its lifespan.
Part 6. How to slow down the battery aging?
While you can’t stop battery aging, you can certainly slow it down with some practical steps:
- Keep It Cool: Avoid exposing your battery to high temperatures. Store your devices in a cool, dry place.
- Avoid Overcharging: Once your battery is fully charged, unplug it to prevent overcharging.
- Partial Discharges: It’s better to recharge your battery before it drops below 20%. Avoid letting it run down to zero.
- Use Quality Chargers: Stick to chargers recommended by the device manufacturer to ensure compatibility and efficiency.
- Proper Storage: If you’re not using a device for a while, store the battery at around 50% charge in a cool environment.
- By following these tips, you can extend the life of your battery and maintain its performance for a longer period.
Part 7. Can the aged battery still be used?
Yes, an aged battery can still be used, but with some caveats. Its performance will be reduced, meaning it won’t hold a charge as long and might be less reliable. This might be acceptable for everyday tasks on non-critical devices. However, for critical devices or applications where performance is crucial, it might be wise to replace the aging battery. Always consider the balance between performance and safety when deciding whether to continue using an aged battery.
In conclusion, Lithium-ion batteries will inevitably age during use. Reducing the degree of aging and extending the safe service life of the battery still requires continuous exploration of the mechanism behind aging and continuous upgrading of materials. By understanding the process, recognizing the signs, and taking steps to slow it down, you can get the most out of your batteries. And while aged batteries can still serve a purpose, knowing when to replace them is key to maintaining optimal performance and safety.
Related Tags:
More Articles

What Battery Powers Your GoPro?
Learn all about GoPro batteries - compatibility, runtime, charging tips, and how to extend battery life for longer shoots.
What Is a Disk Battery? A Simple Guide for Non-Tech Users
A disk battery is a small, round cell used in watches, remotes, and other electronic devices. It delivers steady power for compact, low-drain devices.
What Battery Powers a Space Heater?
Discover the type of battery that powers space heaters and learn how to choose the right one for efficient heating in your home or office.
What Is an LR14 Battery? Learn About This C-Size Cell
The LR14 battery, also known as a C battery, delivers steady power. Learn its specs, uses, lifespan, and how it compares to other battery types.
Watch Battery Dimensions Chart: Sizes, Voltages, and Equivalents Explained
Understanding watch battery dimensions helps you choose the right size, voltage, and equivalent model to keep your watch running safely and smoothly.