Choosing the correct battery—whether high‑power silver‑zinc or long‑life lithium‑ion—requires understanding key trade‑offs in energy density, cycle life, cost and safety.
Part 1. Overview of rechargeable battery technologies
Rechargeable batteries power everything from tiny medical implants to electric aircraft. Over past decades, silver‑zinc and lithium‑ion chemistries have emerged as industry leaders in high‑performance applications. Understanding their trade‑offs—cost, energy density, safety, lifespan and environmental footprint—empowers engineers, purchasing managers and end users to choose the optimal cell for each use case. Below is a quick guide to key decision drivers.
- Cost vs Performance: Total cost per kilowatt‑hour over lifespan.
- Energy Density: Space‑ and weight‑constrained designs (wearables, drones).
- Cycle Life: Products with frequent charge cycles (EV, grid storage).
- Safety Requirements: Mission‑critical, medical or military use.
- Temperature Range: Extreme‑environment operation.
Definition: A Silver‑Zinc battery is a rechargeable cell using silver oxide and zinc electrodes, prized for high energy density in aerospace and medical devices. A Lithium‑ion rechargeable battery uses lithium-ion intercalation in carbon anodes and metal-oxide cathodes, offering long cycle life for consumer electronics and EVs.
Key Benefits & Drawbacks at a Glance
- Silver‑Zinc: Ultra‑high power bursts, inherent safety, simple cell design but limited cycles and higher materials cost.
- Lithium‑Ion: Industry‑wide support, proven long life, fast‑charge capability, but requires complex management and thermal safeguards.
Part 2. Silver zinc battery
A Silver Zinc battery is a type of rechargeable battery that consists of a cylindrical or rectangular shape and employs silver oxide and zinc as its primary materials. These batteries, known for their high energy density and reliability, frequently use specialized applications where performance is crucial.
Composition and How They Work
- Cathode: Silver oxide (AgO)
- Anode: Zinc (Zn)
- Electrolyte: Alkaline solution, usually potassium hydroxide (KOH)
When discharging, the zinc anode releases electrons, which flow through the external circuit to the silver oxide cathode. The silver oxide gets reduced to silver, while the zinc oxidizes to zinc oxide. This flow of electrons generates the electric current used to power devices. Recharging reverses this chemical reaction, restoring the battery’s capacity.
Development History
Silver‑zinc cells have early naval applications in the 1950s. Initial research targeted submarines and torpedoes requiring compact, high‑power sources. Over time, improvements in silver oxide purity and separator materials reduced self‑discharge rates from over 20%/month to under 5%/month.
Characteristics
- High energy density provides more power in a smaller size.
- Reliable performance ensures consistent output and efficiency.
- Short lifespan compared to other rechargeable batteries.
- The lightweight design makes it easier to transport and integrate into devices.
- Environmentally friendly due to the use of less toxic materials.
Advantages:
- High energy output makes them suitable for high-drain applications.
- The lightweight and compact design is ideal for portable and space-constrained devices.
- Safe and stable with a lower risk of leakage or explosion.
- They are environmentally friendly since they are easier to recycle and less environmentally harmful.
Disadvantages:
- It is expensive to produce due to the silver content.
- It has a shorter lifespan with a limited number of recharge cycles.
- Maintenance requirements mean they may need more frequent replacement.
Common Applications
- Military equipment uses these batteries in communication devices and other critical tools.
- Aerospace devices rely on them to power instruments and systems in spacecraft.
- Medical devices utilize these batteries in portable medical equipment and implants.
- Hearing aids prefer Silver Zinc batteries for their small size and high power.
- High-performance drones choose them for their lightweight and high-energy needs.
Storage & Maintenance Tips
- Store at 40–60% state‑of‑charge to minimize capacity fade.
- Avoid temperatures above 45 °C; prolonged heat accelerates zinc corrosion.
- Perform shallow discharge cycles monthly when in storage.
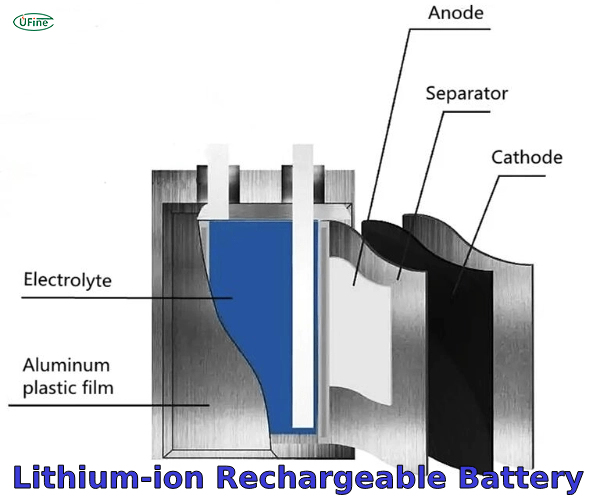
Part 3. Lithium-ion rechargeable battery
A Lithium-ion rechargeable battery, commonly found in cylindrical or prismatic shapes, utilizes lithium ions as the primary charge carriers during discharge and recharge cycles. Various electronic devices widely use these batteries due to their high energy density and long cycle life.
Composition and How They Work
- Anode: Typically made of carbon-based materials like graphite, lithium titanate, or silicon.
- Cathode: Usually composed of lithium metal oxides such as lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), or lithium manganese oxide (LiMn2O4).
- Electrolyte: Lithium salt dissolved in an organic solvent or polymer electrolyte.
Lithium ions move from the anode to the cathode through the electrolyte, creating an electric current that powers the device during discharge. When recharging, the process reverses, with lithium ions moving from the cathode back to the anode.
Characteristics
- High energy density provides longer-lasting power in a compact size.
- Stable voltage output throughout the discharge cycle.
- Longer cycle life compared to other rechargeable batteries.
- Its lightweight design makes it ideal for portable electronics.
- Vulnerable to damage if overcharged or overheated.
Advantages:
- High energy density results in longer run times between charges.
- Lightweight and compact design suitable for portable devices.
- A low self-discharge rate allows for extended shelf life.
- Fast recharge times compared to other battery types.
Disadvantages:
- Susceptible to aging, with reduced capacity over time.
- Risk of thermal runaway and fire if improperly handled or damaged.
- It is more expensive to produce than some other battery types.
- Requires protection circuitry to prevent overcharging and over-discharging.
Environmental Considerations
Manufacturing Lithium‑ion cells consumes significant energy and often involves cobalt mining, raising ethical concerns. Advances in cathode chemistry (e.g., NMC811, LFP) aim to reduce or eliminate cobalt, lowering environmental and social impact.
Common Applications
- Smartphones and tablets utilize lithium-ion batteries because of their high energy density and compact size.
- Laptops and notebooks rely on these batteries for portable power.
- Electric vehicles use large Lithium-ion battery packs for propulsion.
- Power tools, such as cordless drills and saws, benefit from their lightweight and high-energy output.
- Renewable energy storage systems integrate Lithium-ion batteries to store electricity generated from solar panels or wind turbines.
Future Trends
- Solid‑State Electrolytes: Promise ultra‑high energy density and zero flammability.
- Silicon‑Dominant Anodes: Enabling >400 Wh/kg cell energy.
- Recycling Innovations: Closed‑loop recovery targeting >90% material reuse.
Part 4. Comparing silver zinc batteries and lithium-ion rechargeable batteries
| Parameter | Silver‑Zinc | Lithium‑Ion |
|---|---|---|
| Energy Density (Wh/kg) | 100–150 | 150–250 |
| Cycle Life (cycles) | 150–300 | 500–1000+ |
| Nominal Voltage | 1.5 V | 3.6–3.7 V |
| Charge Rate | 0.2 C–0.5 C | 1 C–2 C (fast charge) |
| Recycling | Easy; less toxic | Challenging; cobalt & lithium recovery |
Use‑Case Comparison
- Medical Implants: Silver‑zinc preferred for low voltage, high reliability and biocompatibility.
- Consumer Electronics: Lithium‑ion dominates with ultra‑thin form factors and >1,000 cycles.
- Aerospace & Defense: Silver‑zinc chosen when mission‑critical safety outweighs cycle life.
- Electric Mobility: Lithium‑ion selected for cost per cycle and fast charging infrastructure.
Part 5. FAQs about silver zinc and lithium-ion rechargeable batteries
Are zinc batteries better than lithium‑ion batteries?
While zinc batteries offer enhanced safety and lower production cost, lithium‑ion batteries deliver higher energy density and longer cycle life (typically 500–1,000+ cycles).
Will zinc batteries replace lithium‑ion batteries?
Zinc batteries are unlikely to fully replace lithium‑ion due to their shorter cycle life (150–300 cycles) and higher cost per cycle; they instead complement lithium‑ion in safety‑critical and high‑power applications.
Are silver‑zinc batteries rechargeable?
Yes, silver‑zinc batteries can be recharged for approximately 150–300 cycles before capacity drops below 80%.
How long do zinc batteries last?
Under standard conditions, zinc batteries have a shelf life of 3–5 years and maintain performance for 150–300 charge/discharge cycles.
Related Tags:
More Articles

High‑Capacity 3S LiPo Batteries: 5000 mAh vs. 10000 mAh
Compare 3S LiPo 5000mAh vs 10000mAh batteries by weight, power, and use. Find the best fit for your drone, RC car, or boat setup.
Top 5 Applications for Small 3S LiPo Batteries
Small 3S LiPo batteries power drones, RC gear, wearables, and robotics with high energy and low weight. Making them ideal for compact electronics projects.
Building and Charging Your Own 3S LiPo Pack: A Step‑by‑Step Guide
Learn how to build, balance, and charge a 3S LiPo battery pack safely at home with this complete DIY guide for hobbyists and beginners.
How to Choose the Right LiPo Battery Plug Type?
Discover the best LiPo battery plug types, how to choose them, and expert tips for safe usage, soldering, and maintenance.
Choosing the Right Connector for Your 3S LiPo Battery
Choosing the right 3S LiPo connector depends on current, space, and use. Learn the pros and cons of XT60, JST, EC3, and more.