- Part 1. What is an aluminum air battery?
- Part 2. Why consider aluminum air batteries over lithium-ion?
- Part 3. What materials are needed to build an aluminum air battery?
- Part 4. How do you assemble an aluminum air battery step-by-step?
- Part 5. What are the chemical reactions inside an aluminum-air battery?
- Part 6. How efficient is an aluminum air battery?
- Part 7. What are the main challenges in aluminum air battery design?
- Part 8. How can the lifespan of an aluminum air battery be increased?
- Part 9. Can aluminum air batteries be used in electric vehicles?
- Part 10. What are the future trends in aluminum air battery technology?
- Part 11. FAQS about the aluminum-air battery
What is an aluminum air battery, and how does it work? An aluminum air battery is an energy storage device that uses aluminum as an anode and oxygen from the air as a cathode. It generates electricity through a chemical reaction between aluminum and oxygen, producing aluminum hydroxide as a byproduct. This battery type is lightweight, has high energy density, and is often considered a promising alternative to lithium-ion batteries.
In this guide, we’ll explore everything from the materials used to the step-by-step assembly and efficiency tips to help you get the most out of your aluminum air battery design. Whether you’re a hobbyist, student, or engineer, this comprehensive article provides a clear and engaging overview.
Part 1. What is an aluminum air battery?
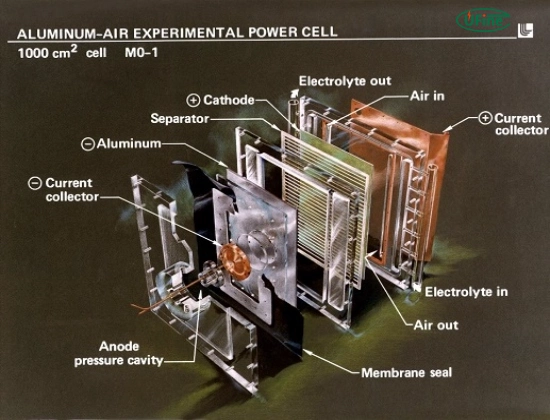
An aluminum air battery is a metal-air electrochemical cell. Unlike traditional batteries that store both anode and cathode materials internally, this battery draws oxygen from the air, making it lighter and more energy-dense.
The key components include:
- Aluminum anode (usually pure or alloyed)
- Air cathode (often made of a porous carbon structure with a catalyst)
- Electrolyte (usually a saltwater or alkaline solution)
When the battery operates, aluminum oxidises and releases electrons, which flow through the external circuit, providing power.
Part 2. Why consider aluminum air batteries over lithium-ion?
Here’s a quick comparison that might surprise you:
| Feature | Aluminum Air Battery | Lithium-Ion Battery |
|---|---|---|
| Energy density | Higher (up to 1300 Wh/kg) | Moderate (150–250 Wh/kg) |
| Weight | Lightweight | Heavier |
| Cost | Cheaper material (aluminum) | Expensive materials (lithium, cobalt) |
| Reusability | Single-use (but recyclable) | Rechargeable |
| Eco-friendliness | Less toxic | More toxic components |
The aluminum air battery is beautiful for electric vehicles and emergency backup systems where high energy density and weight reduction are critical.
Part 3. What materials are needed to build an aluminum air battery?
To build your aluminum air battery, you’ll need the following materials:
- Aluminum sheet or foil (acts as the anode)
- Activated carbon or carbon cloth (for the air cathode)
- Electrolyte solution (usually salt water or potassium hydroxide)
- Separator (paper towel or porous membrane)
- Conductive wire (to connect the electrodes)
- Current collector (such as nickel mesh or stainless steel)
- Plastic or acrylic casing (to hold everything together)
Ensure all components are non-corrosive, lightweight, and chemically compatible with your electrolyte.
Part 4. How do you assemble an aluminum air battery step-by-step?
Here’s a beginner-friendly guide to assembling one:
- Cut the aluminum sheet to fit inside your container; this will be your anode.
- Prepare the air cathode by pressing activated carbon into a porous material.
- Soak the separator in the electrolyte and place it between the anode and cathode.
- Place the electrodes inside the plastic container, ensuring they do not touch directly.
- Use wires to connect the anode and cathode to a load, such as an LED or small motor.
- Seal the container, leaving an opening for air to reach the cathode.
Tip: Ensure the air cathode faces outward to allow oxygen intake and improve battery efficiency.
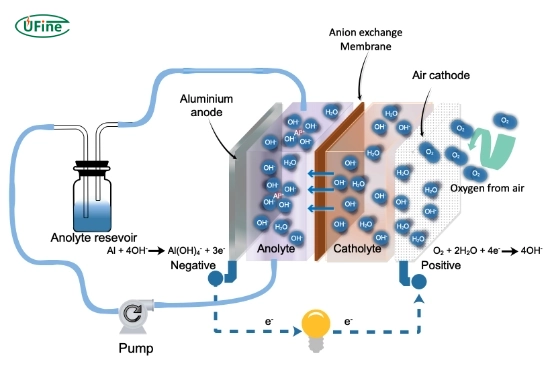
Part 5. What are the chemical reactions inside an aluminum-air battery?
The electrochemical reaction can be summarised as:
At the anode (oxidation):
Al → Al³⁺ + 3e⁻
At the cathode (reduction):
O₂ + 6H₂O + 4e⁻ → 4OH⁻
Overall reaction:
4Al + 3O₂ + 6H₂O → 4Al(OH)₃
This reaction produces aluminum hydroxide, accumulating in the electrolyte and may need to be removed or managed for long-term use.
Part 6. How efficient is an aluminum air battery?
Aluminum-air batteries have a high theoretical energy density, but practical efficiency depends on the following:
- Purity of aluminum
- Design of the air cathode
- Type of electrolyte
- Internal resistance and electrode spacing
Efficiency can be around 50–70% for well-designed prototypes, but commercial models may vary—Utilise high-surface-area electrodes and fresh electrolytes to enhance performance and prevent cathode clogging.
Part 7. What are the main challenges in aluminum air battery design?
Despite their promise, these batteries face several challenges:
- Short lifespan due to aluminum corrosion
- Byproduct buildup (aluminum hydroxide)
- Cannot be recharged traditionally (need to replace the aluminum)
- Air cathode drying out or becoming blocked
- Limited power output for high-drain applications
Overcoming these issues often involves better materials and smart design tweaks.
Part 8. How can the lifespan of an aluminum air battery be increased?
Here are some practical tips:
- Use high-purity aluminum to reduce self-corrosion
- Add corrosion inhibitors to the electrolyte
- Use a flow system to remove aluminum hydroxide
- Keep the air cathode moist but breathable
- Use modular designs for easy anode replacement
Pro tip: A hybrid system using aluminum air for energy storage and a small lithium-ion battery for bursts of power can work well.
Part 9. Can aluminum air batteries be used in electric vehicles?
Yes, but with limits. Aluminum-air batteries are perfect for long-range energy storage. Still, they cannot handle frequent charging cycles like lithium-ion batteries.
Some car companies are exploring hybrid systems, where aluminum air batteries provide long-distance energy and lithium-ion handles acceleration and braking. The aluminum can be swapped at service stations, similar to refuelling.
Part 10. What are the future trends in aluminum air battery technology?
The future looks bright with innovations like:
- Rechargeable aluminum air systems using special electrolytes
- 3d-printed components for custom battery shapes
- Nanotechnology to boost air cathode performance
- Recycled aluminum for sustainable production
- Mass production in aerospace, defence, and EV industries
If these trends continue, aluminum air batteries could reshape how we store and use energy.
Artikel Terkait: The Chemistry Behind Aluminum-Ion Batteries: How It Works and Why It Matters
Part 11. FAQS about the aluminum-air battery
Is an aluminum air battery rechargeable?
Not in the traditional sense. You can’t plug it in to recharge. Instead, you replace the aluminum and refresh the electrolyte.
What is the energy density of aluminum air batteries?
Up to 1300 Wh/kg is significantly higher than most lithium-ion batteries.
How long do aluminum air batteries last?
Depending on usage, they can run for several days to a few weeks. Still, the aluminum anode must eventually be replaced.
Can I build an aluminum air battery at home?
Yes! You can build a simple version for educational use with basic materials, such as aluminum foil, saltwater, carbon cloth, and a container.
Are aluminum air batteries safe?
Generally yes. They don’t explode or catch fire like lithium batteries. However, the electrolyte can be corrosive, so handle it with care.
Related Tags:
More Articles

Lithium-Ion vs Lead-Acid AMR & AGV Batteries Compared
Discover the pros and cons of lithium-ion and lead-acid AMR & AGV batteries. Learn about cost, lifespan, safety, and which is right for your fleet.
Robot Vacuum Battery Replacement: Easy Step-by-Step Guide
Learn how to replace a robot vacuum battery safely and easily. Step-by-step instructions, battery types, costs, and common mistakes to avoid.
Discover how to choose the right battery for your robot. Compare Li-ion, LiFePO₄, NiMH, and more for performance, safety, and cost.
Inside Humanoid Robot Battery Pack Design
A deep dive into humanoid robot battery pack design, covering battery life, voltage, capacity, safety, and real-world engineering trade-offs.
Humanoid Robot Battery Life: How Long Do They Really Last?
Most humanoid robots run 1.5–4 hours per charge. Learn real-world battery life, battery types, capacity limits, and future improvements.